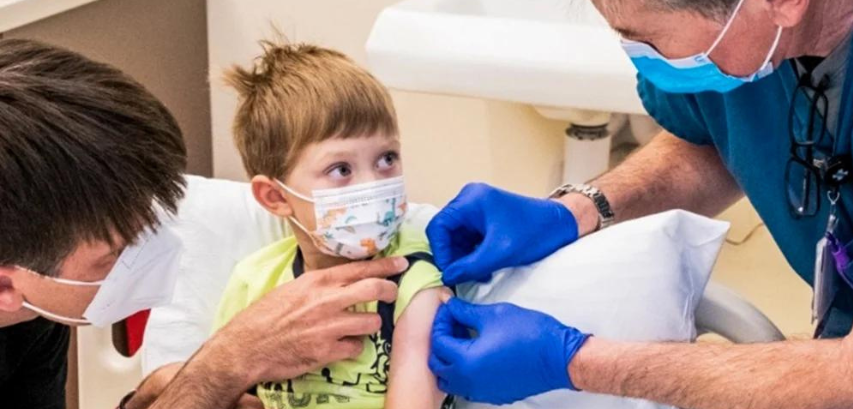
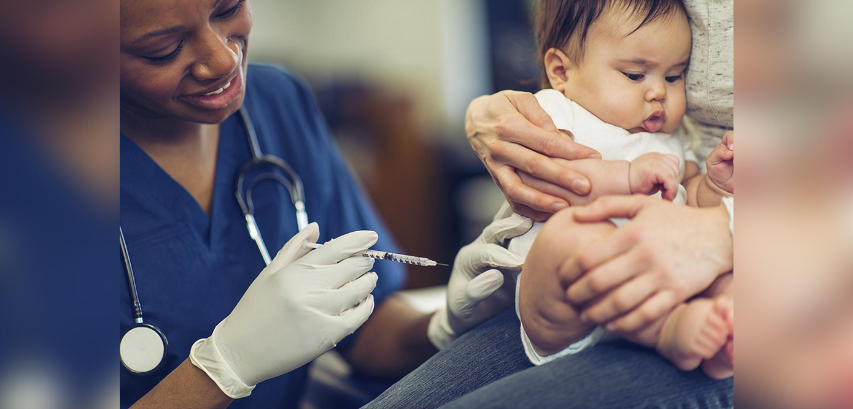
While the first doses of the Covid vaccine were widely administered in adults over a year ago in the spring of 2021, with children over 5 finally receiving vaccines in the fall and winter of last year as well, the time has come for the youngest of our population to finally be protected against the Covid virus that has plagued us for over two years now. Following the official FDA approval, children between the ages of 6 months and five years are now eligible to be vaccinated with either the Moderna or Pfizer vaccine, with three doses for Pfizer and two for Moderna. Children receiving Pfizer will be given three doses of 3 micrograms, compared to the two doses of 30 micrograms that adults get.
Tag: Moderna
Moderna and Pfizer Allow 6 Month Old
Babies To Get COVID-19 Vaccine
The Food and Drug Administration’s committee of independent vaccine experts voted Wednesday to recommend authorizing both Pfizer and Moderna COVID-19 vaccines for children up to 6 months or older. Moderna’s vaccine is for children ages 6 months to 5 years, while Pfizer’s is for children ages 6 months to 4 years.
President Biden Urges Congress to Approve COVID Spending Bill
President Biden addressed Congress in a speech at the White House on Wednesday, warning them that U.S. progress against the spread of COVID-19 would be at severe risk if Congress does not approve a huge spending bill for emergency coronavirus relief aid.
The CDC Signs Off on Second COVID-19 Shot for People 50+
On Tuesday, March 29th, the CDC and other U.S. regulators approved and authorized a second COVID-19 booster shot for individuals 50 years old and older, providing more protection for individuals more vulnerable. Individuals over the age of 50 can now receive either the Pfizer-BioNTech or the Moderna Covid-19 vaccine.
Johnson and Johnson Covid-19 Booster Shot on The Horizon
Johnson and Johnson are the third and final company with a federally backed Corona Virus vaccination that plans to ask the FDA to approve their booster shot.
Another Pfizer vaccine booster is in the works
Melody Smith
On Thursday, Pfizer announced that it is currently developing a third booster shot for those who have already been vaccinated for COVID. This announcement comes as the highly transmissible Delta variant becomes an increased risk. In many countries, it is becoming the dominant strain, and this pattern is likely to continue. Pfizer said that its third booster shot would specifically target the Delta variant.
Moderna Applies for Full FDA Approval of COVID Vaccine
On Tuesday, pharmaceutical company Moderna announced that they would be seeking full approval from the Food and Drug Administration (FDA) for their COVID-19 vaccine. The company said in a press release that they had asked the FDA for this approval but that the process of actually getting it fully approved is expected to take months. This news comes almost a week after fellow drug company Pfizer announced that they would be applying for full approval of their COVID-19 vaccine.
Moderna Set to be Approved for Teens
A month after the Pfizer vaccine was approved for children aged 12-17, Moderna announced that it is close to being approved as well. Moderna released a study Tuesday suggesting that its COVID-19 vaccine was 100% effective in a study conducted with adolescents. The pharmaceutical giant announced that they plan to ask the FDA to expand emergency use of the vaccine to this age group by as early as June.